The solubility, permeability, and absorptivity of the active pharmaceutical ingredients (APIs) have always been key for targeted and precise treatment when preparing and producing Oral Suspensions, Release Tablets, and more.

The solubility, permeability, and absorptivity of the active pharmaceutical ingredients (APIs) have always been key for targeted and precise treatment when preparing and producing Oral Suspensions, Release Tablets, and more.

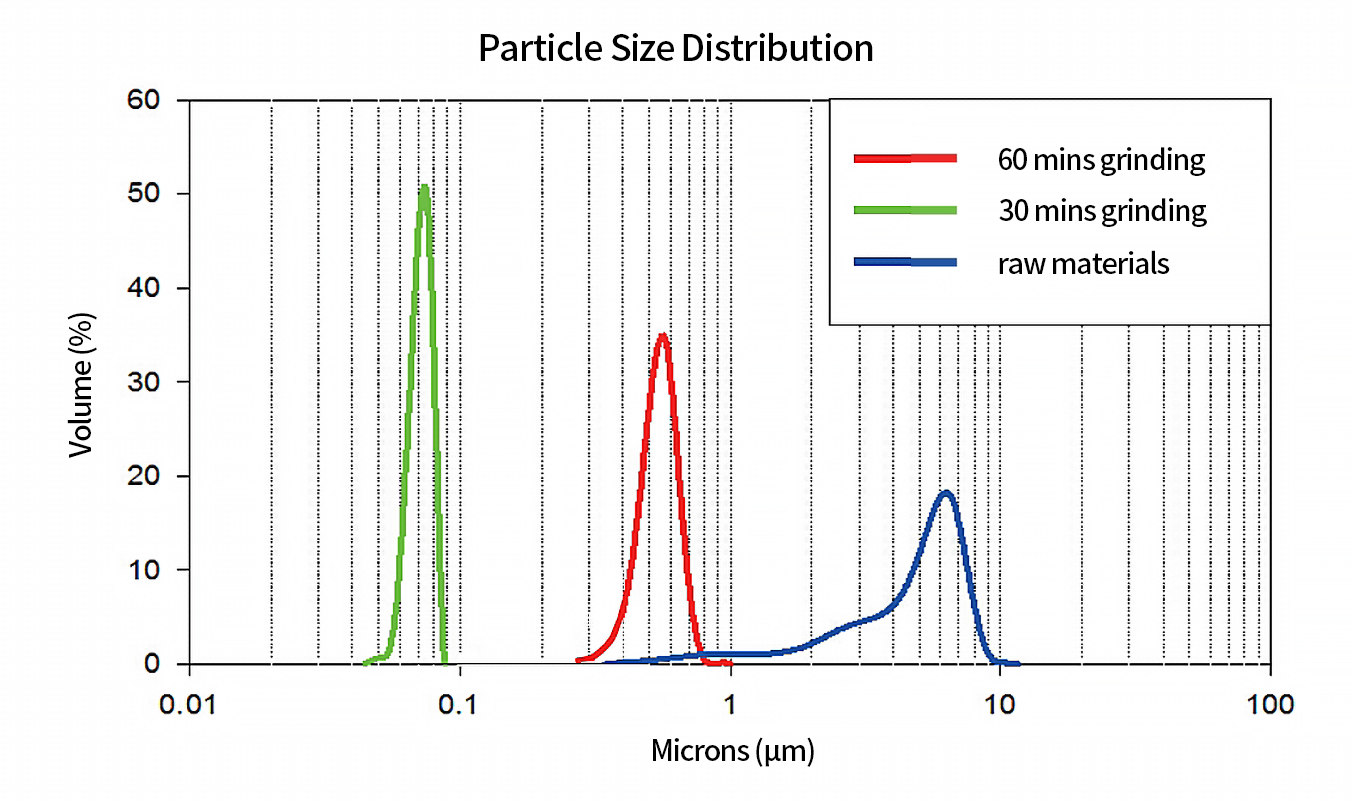
Wet milling technology, for example, using the bead mill, can reduce the particle size in a liquid medium (typically water or a solvent) so the surface contact area of APIs is increased. This leads to a high dissolution rate and absorption, especially for those poorly soluble ingredients whose bioavailability is limited when administered at larger particle sizes. A uniform particle size distribution can also be achieved. The active pharmaceutical ingredients are usually mixed or dissolved in other excipients or components. The smaller particles evenly distributed can ensure the drug dose has the desired therapeutic performance, thereby maintaining dose accuracy and consistency in efficacy.

To provide a sterile production for APIs, LONGLY Group serial smart equipment and grinding solutions comply with Cleaning-in-Place (CIP) and Sterilization-in-Place (SIP) covering laboratory application to industrial production to meet your specific demand.
